DePeaux K, Rivadeneira DB, Lontos K, Dean VG, Gunn WG, Watson MJ, Yao T, Wilfahrt D, Hinck C, Wieteska L, Thorne SH, Hinck AP, and Delgoffe GM, An oncolytic virus–delivered TGFβ inhibitor overcomes the immunosuppressive tumor microenvironment. J. Exp. Med. 2023 Vol. 220 No. 10 e20230053


Li F, Sheng Y, Hou W, Sampath P, Byrd D, Thorne S, Zhang Y (2020) CCL5-armed oncolytic virus augments CCR5-engineered NK cell infiltration and antitumor efficiency. J Immunother Cancer. 8(1):e000131 PMID: 32098828

Rivadeneira DB, DePeaux K, Wang Y, et al. Oncolytic Viruses Engineered to Enforce Leptin Expression Reprogram Tumor-Infiltrating T Cell Metabolism and Promote Tumor Clearance. Immunity. 2019;51

Rivadeneira DB, DePeaux K, Wang Y, Kulkarni A, Tabib T, Menk AV, Sampath P, Lafyatis R, Ferris RL, Sarkar SN, Thorne SH, Delgoffe GM (2019) Oncolytic Viruses Engineered to Enforce Leptin Expression Reprogram Tumor-Infiltrating T Cell Metabolism and Promote Tumor Clearance. Immunity. 51(3):548-560. PMID: 31471106

Shao L, Hou W, Scharping NE, Vendetti FP, Srivastava R, Roy CN, Menk AV, Wang Y, Chauvin JM, Karukonda P, Thorne SH, Hornung V, Zarour HM, Bakkenist CJ, Delgoffe GM, Sarkar SN (2019) IRF1 Inhibits Antitumor Immunity through the Upregulation of PD-L1 in the Tumor Cell. Cancer Immunol Res. 7(8):1258-1266. PMID: 31239318
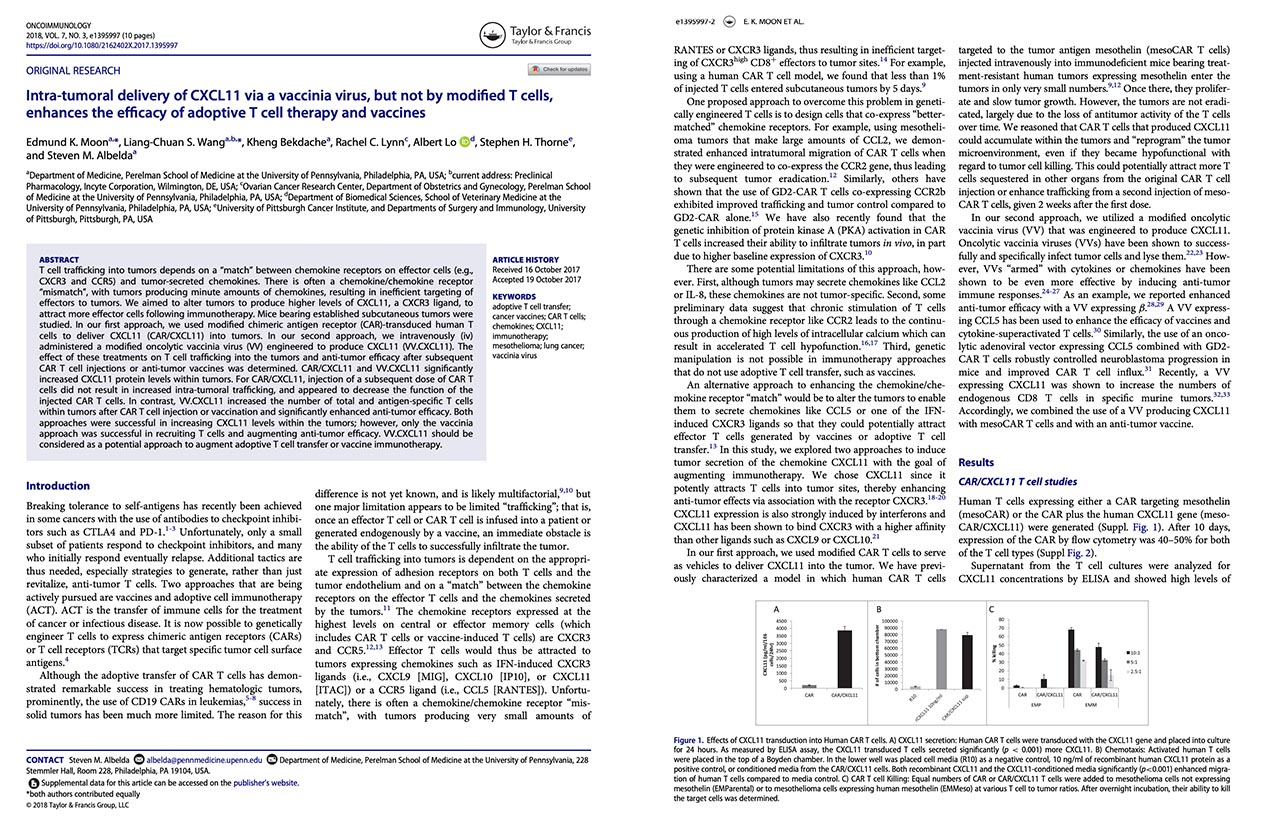
Moon EK, Wang LS, Bekdache K, Lynn RC, Lo A, Thorne SH, Albelda SM. (2018) Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. Oncoimmunology. 7(3): PMID: 29399394

Berkey SE, Thorne SH, Bartlett DL. (2017) Oncolytic Virotherapy and the Tumor Microenvironment. Adv Exp Med Biol. 1036:157-172 PMID: 29275471
Berkey SE, Thorne SH, Bartlett DL. (2017) Oncolytic Virotherapy and the Tumor Microenvironment. Adv Exp Med Biol. 1036:157-172 PMID: 29275471
Thorne SH (2016) Virus fuels NK cell killing of leukemia. Blood. (Epub) PMID 27231392

Thorne SH (2016) Adding STING to the tale of Oncolytic Virotherapy. Trends in Cancer 2(2):67-68 PMID: 27004260

Sampath P, Thorne SH (2015) Novel therapeutic strategies in human malignancy: Combining immunotherapy and oncolytic virotherapy. Oncolytic Virotherapy 4:75-82

Rojas J, Sampath P, Hou W, Thorne SH (2015) Defining Effective Combinations of Immune Checkpoint Blockade and Oncolytic Virotherapy. Clin. Cancer Res. [Epub ahead of print] PMID: 26187615

Zeh H, Downs-Canner S, McCart J, Guo Z, Rao U, Ramalingam L, Thorne S, Jones H, Kalinski P, Wieckowski E, O’Malley M, Daneshmand M, Hu K, Bell J, Hwang T, Moon A, Breitbach C, Kirn D, Bartlett D (2014) .First-in-man Study of Western Reserve Strain Oncolytic Vaccinia Virus: Safety, Systemic Spread and Anti-tumor Activity. Mol Ther.

Thorne SH. (2014). Immunotherapeutic potential of oncolytic vaccinia virus. Front Oncol. 4:155. PMID: 24987615

Hou W, Chen H, Rojas J, Sampath P, Thorne SH (2014) Oncolytic Vaccinia Virus Demonstrates Anti-angiogenic Effects Mediated by Targeting of VEGF. Int J Cancer. 2014 [Epub ahead of print] PMID: 24474587

Albelda SM, Thorne SH. (2014) Giving Oncolytic Vaccinia Virus More BiTE. Mol Ther. (1):6-8. PMID: 24384909

Sampath P, Thorne SH. (2013) Arming viruses in multi-mechanistic oncolytic viral therapy: current research and future developments, with emphasis on poxviruses. Oncolytic Virotherapy. 3:1-9 PMID

Yan W, Chang Y, Liang X, Cardinal JS, Huang H, Thorne SH, Monga SP, Geller DA, Lotze MT, Tsung A. (2012) High mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012 Jan 11 [Epub ahead of print] PMID: 22234969

Thorne SH. (2011) Next-generation oncolytic vaccinia vectors. Methods Mol Biol. 2012;797:205-15. PMID: 21948478
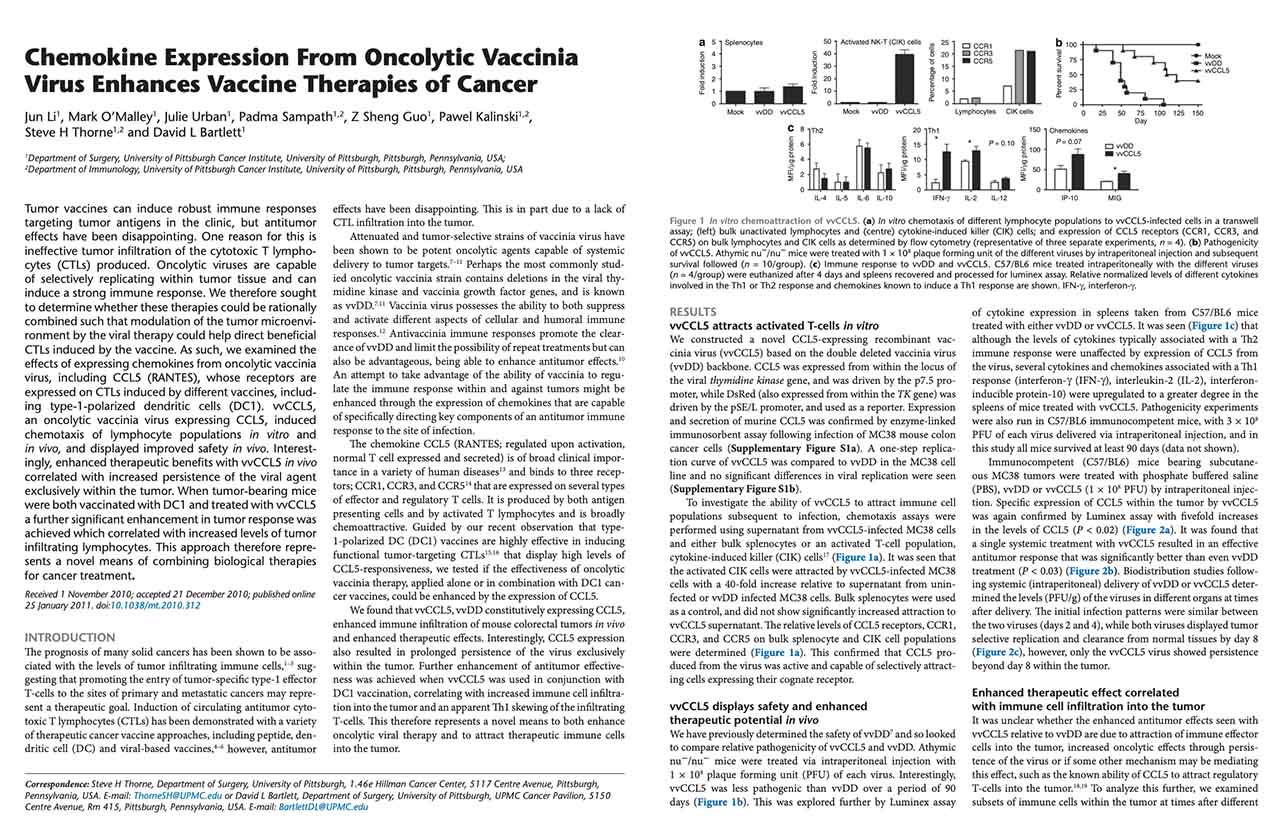
Li J, O’Malley M, Urban J, Sampath P, Guo ZS, Kalinski P, Thorne SH, Bartlett DL. (2011) Chemokine Expression From Oncolytic Vaccinia Virus Enhances Vaccine Therapies of Cancer. Mol Ther. Jan 25. [Epub] PMID: 21266959
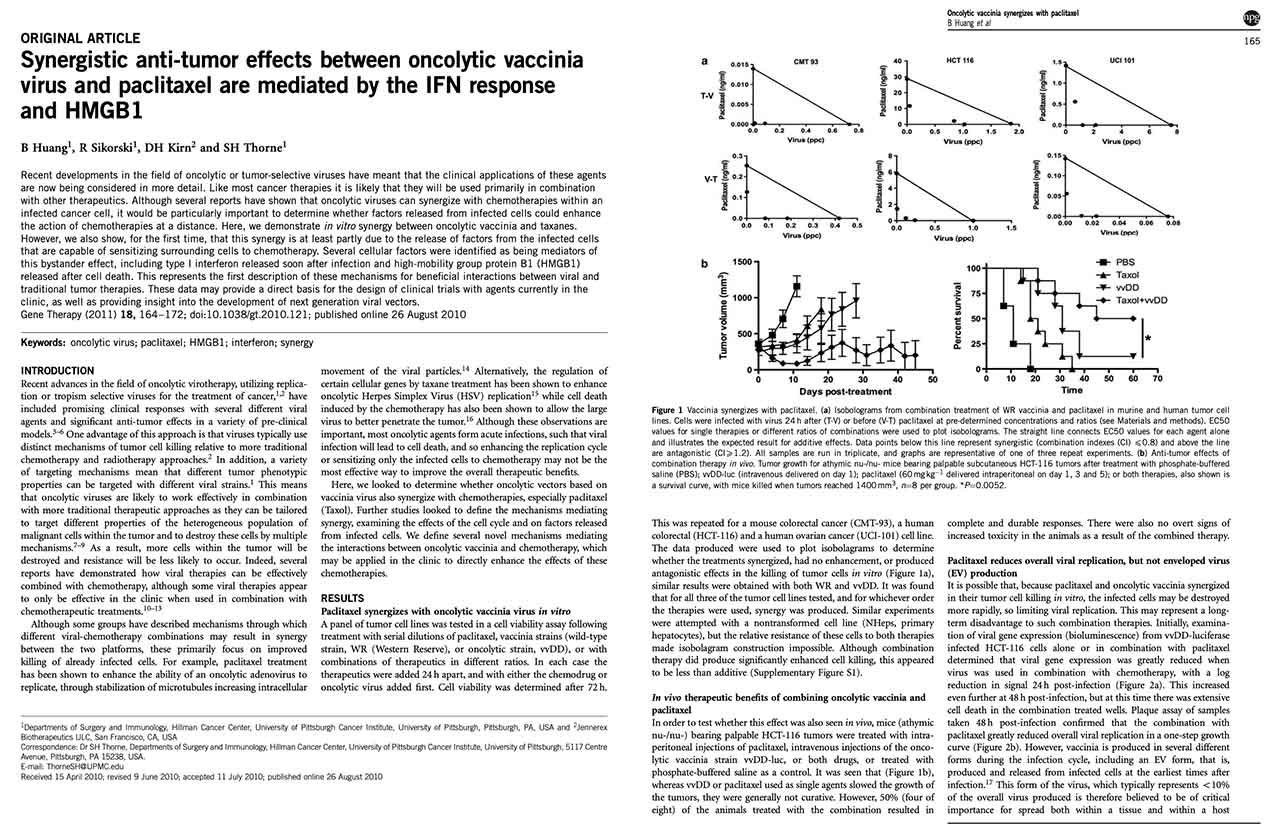
Huang B, Sikorski R, Kirn DH, Thorne SH. (2010) Synergistic anti-tumor effects between oncolytic vaccinia virus and paclitaxel are mediated by the IFN response and HMGB1. Gene Ther. 2010 Aug 26. [Epub] PMID: 20739958

Le Boeuf F, Diallo JS, McCart JA, Thorne S, Falls T, Stanford M, Kanji F, Auer R, Brown CW, Lichty BD, Parato K, Atkins H, Kirn D, Bell JC (2010). Synergistic Interaction Between Oncolytic Viruses Augments Tumor Killing. Mol Ther. 2010 Mar 16. [Epub] PMID: 20234341

Thorne SH (2009) Design and testing of novel oncolytic vaccinia strains. Methods Mol Biol. 542: 635-647

Kirn DH, Thorne SH. (2009) Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer 9(1): 64-71

Kirn DH, Wang Y, Liang W, Contag CH, Thorne SH (2008) Enhancing poxvirus oncolytic effects through increased spread and immune evasion. Cancer Res. April 68(7): 2071-5

Thorne SH, Hermiston T, Kirn D (2005). Oncolytic Virotherapy: Approaches to Tumor Targeting and Enhancing Antitumor Effects. Sem Onc. Dec:32:537-548