HOW ONCOLYTIC VIRAL IMMUNOTHERAPY WORKS
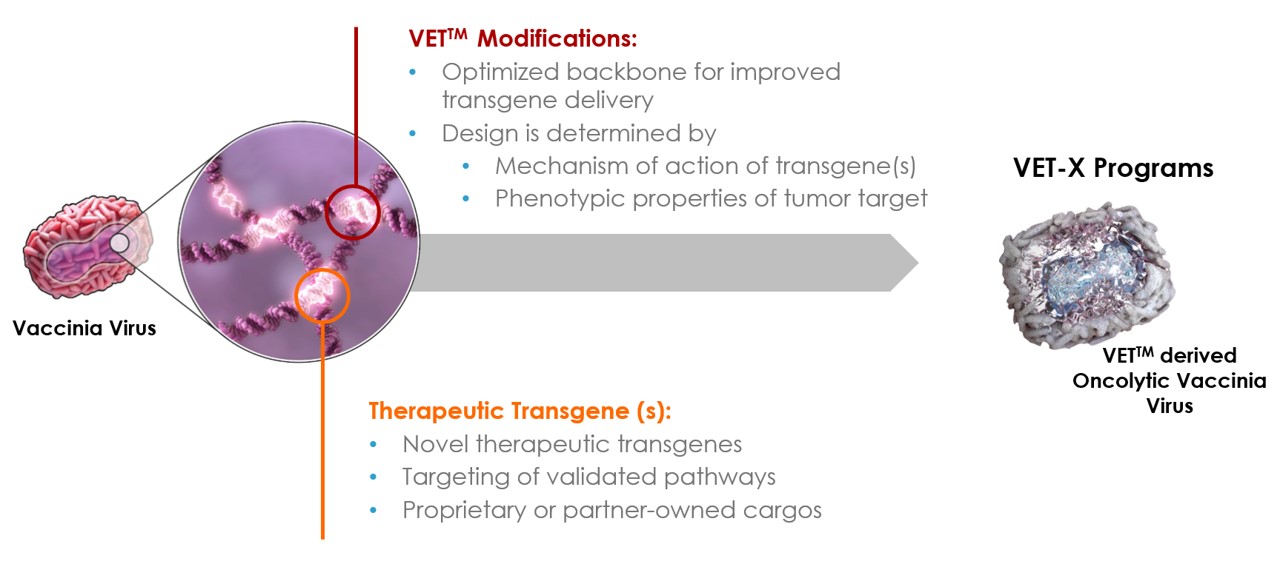
Oncolytic viral immunotherapy utilizes engineered viruses that can preferentially infect and kill cancer cells thereby generating de novo or boosting pre-existing anti-tumor immunity to combat cancer. Most oncolytic viruses are genetically modified to enhance tumor tropism or tumor targeting abilities, so that the virus’ virulence against non-tumor cells are significantly reduced. This allows the virus to replicate preferentially in tumor cells and leave normal cells unharmed.
In addition to direct cell killing abilities, oncolytic viruses can also stimulate a pro-inflammatory environment within the host tumor as well as enhancing antigen release and recognition. This immune activation can counteract the immune evasiveness of tumors. In essence, the virus stimulates a systemic immune response that can combat cancer.